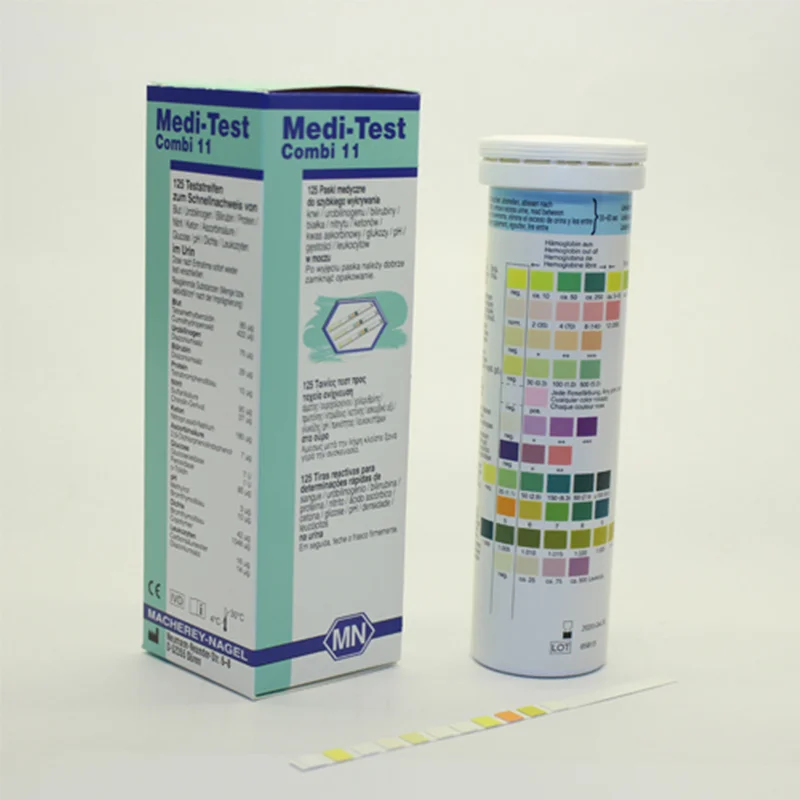
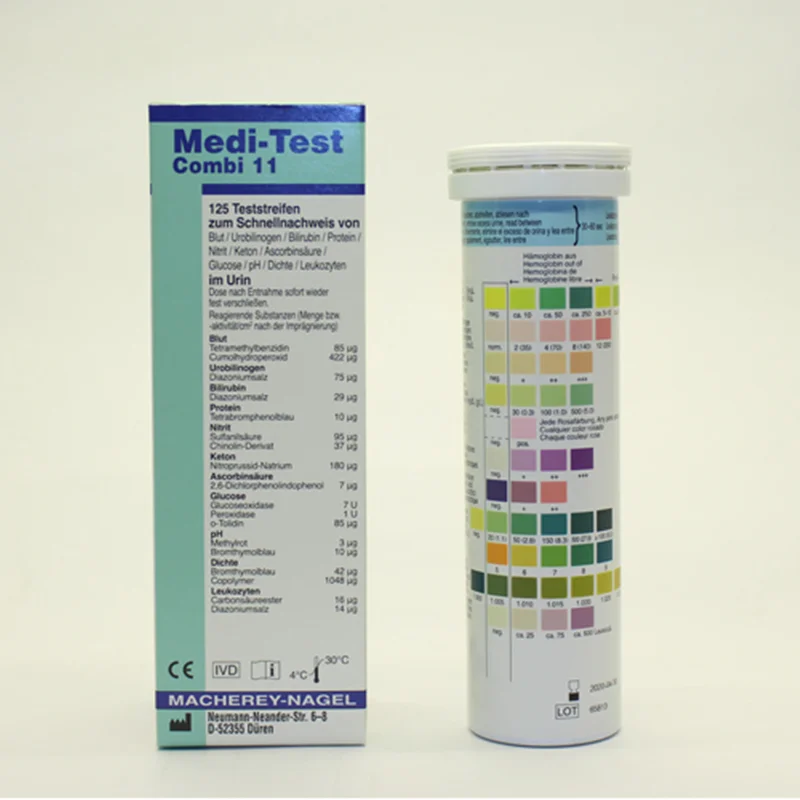
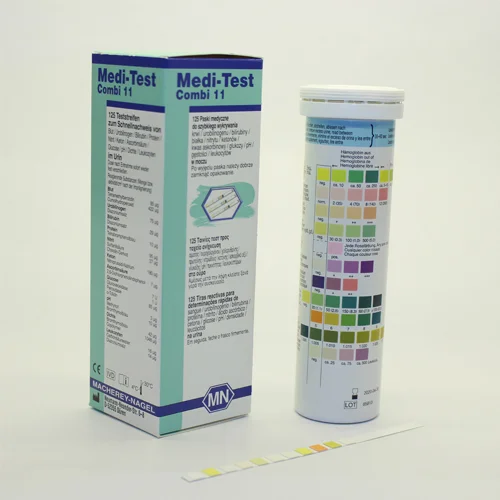
The use of urine test strips is acknowledged as modern screening method in medical practice. Within minutes important information on the health status of the patient is obtained. This simplifies the decision about further diagnostic and therapeutic action.
Related
The use of urine test strips is acknowledged as modern screening method in medical practice. Within minutes important information on the health status of the patient is obtained. This simplifies the decision about further diagnostic and therapeutic action.
| Platform | Urine test strips |
| Brand | Medi-Test |
| Parameter | Ascorbic acid, Bilirubin, Blood, Density, Glucose, Ketones, Leukocytes, Nitrite, pH, Protein, Urobilinogen |
| Ascorbic acid | neg • + • ++ |
| Bilirubin | neg • + • ++ • +++ |
| Blood | neg • ca. 5–10 • ca. 50 • ca. 250 Ery/µL |
| Density | 1.000 • 1.005 • 1.010 • 1.015 • 1.020 • 1.025 • 1.030 |
| Glucose | neg • norm • 50 • 150 • 500 • ≥1000 mg/dL |
| Ketones | neg • + • ++ • +++ |
| Leukocytes | neg • ca. 25 • ca. 75 • ca. 500 Leuco/µL |
| Nitrite | neg • pos |
| pH | 5 • 6 • 7 • 8 • 9 |
| Protein | neg. • 30 • 100 • 500 mg/dL |
| Urobilinogen | norm • 2 • 4 • 8 • 12 mg/dL |
| Evaluable on reflectometer | No |
| Shelf life (from production) | 2 Years |
| FDA 510(k) | K991927, Complexity Waived |
| Hazardous material | No |
| CE certified | CE-certified according to IVD Directive 98/79/EC, for professional use. |
| Storage temperature | 4−30 °C |
| Scope of delivery | 125 test strips in a tube, instructions for use |
Product Name
Medi‑Test Combi 11
Cat. No.
930871
Package unit
125 Tests
Company
Macherey-Nagel
Origin
Germany
Storage Temperature
4−30 °C
Certified
CE-certified according to IVD Directive 98/79/EC, for professional use
FDA
FDA 510(k)
K991927, Complexity Waived